Back to the rolled gold wire- Mitch over at Hickoree's Hard Goods asked to make it into a pretzel pendant.
(...gotta get me a Hill-side tie...)
Yes, the gold is a weird colour, but dont worry about it. ( its just that marble dust I was talking about)
A Pretzel Needs Salt.
So Im going to put some tiny gold granules on there.
Granulation is an ancient technique, mastered by the Etruscans. Seems to be a bit controversial as to how it actually works- but this is how it was taught to me by Dr Robert Baines:
Making the granules:
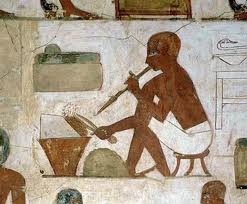
Place gold filings on layers of charcoal dust.
Heat this up, and the bits will melt into spheres; granules.
The dust helps to keep them separate. Then wash off the dust.
It'll be tricky to attach these things to the pretzel, they're really tiny. Smaller than this: "."
Ancient method:
-malachite powder (see pic above)
-organic glue
(Theophilus Presbyter prescribes quince pips, I think? or gum tragacanth is also good. I used a Uhu stick! Seemed to work- felt a little irreverent tho')
-flux
You paste the granules on with a little of this mix.
Heat it up gently with a bushy flame. The glue burns off ( creating a local reducing atmosphere)
Then there's this critical moment when you get a little flash of the metal just before you melt everything.
(stop before that)
The tiny amount of copper in the malachite powder melts with the gold just where the spheres meet the substrate. A very discrete join.( Is it diffusion bonding? Or is it just fusion?)
A weird pic, yes, the pretzel is on the right with some of the granules bonded nicely. See how they are still spherical? ( bit fuzzy, sorry) Good Job!
Ironically, I decided that I was being too technical- the granules were too good, too spherical- not 'baked-in-salty' enough- so I decided to melt them in a bit, undoing my fancy-pants granulation.
The pretzel is ≈ 6mm long.
See, I told you the black stuff would come right off.